Healthcare stocks have a reputation of keeping investors on their toes. The possibility of surging price action, or conversely, earth shattering drops is very real, but these movements can come and go in the blink of an eye.
As a result, these stocks need constant monitoring ahead of key catalysts, as they can affect the price dramatically. Additionally, the price movement is usually dependent on one of the following: either a regulatory approval or trial data. Once either is announced to the public, these stocks either kiss the sky or moan painfully as they crash into the ground. Any healthcare investor must be vigilant, as the rise or drop can occur in minutes.
With this in mind, we set out on our own search to find compelling plays in this volatile industry. Given the sheer size of the market, we used TipRanks’ database to uncover two healthcare stocks with catalysts coming up in October. Each of these Buy-rated tickers has attracted significant praise from analysts, and boasts lofty upside potential. Here’s the scoop.
Avadel Pharmaceuticals (AVDL)
Dublin-based Avadel is specialty pharmaceutical company, with a focus on the treatment of narcolepsy, or excessive daytime sleepiness. The company estimates that there are up to 170,000 diagnosed patients in the US alone, making a significant potential patient base for an approved drug. Avadel’s key drug candidate, FT218, a formulation of sodium oxybate, makes use of a proprietary drug delivery technology for once-daily dosing at bedtime for the treatment of adults.
FT218’s single-dosing feature differentiates it from currently available oxybates, which require dosing twice nightly. This is a serious drawback for patients who have difficulties with interrupted sleep. Avadel’s formulation aims to eliminate this problem.
Last year, Avadel reported the successful completion of the REST-ON Phase 3 clinical trial of FT218 for the treatment of EDS (excessive daytime sleepiness) and cataplexy in patients suffering from narcolepsy, which demonstrated statistically and clinically meaningful results in meeting three key endpoints. The drug is currently undergoing the open-label RESTORE clinical study, and has received orphan drug designation from the FDA.
The final step before commercialization of FT218 can commence is regulatory approval, and Avadel got that process started in February of this year, with the New Drug Application (NDA) filing with FDA. The Agency’s review process is nearing completion, and the PDUFA date – when the FDA will announce its decision – is set for this coming October 15.
There remains one potential stumbling block for Avadel’s FT218 program. In May of this year, Jazz Pharmaceuticals sued Avadel for copywrite infringement. Jazz also is working on a one-nightly dosed narcolepsy treatment; however, Avadel’s patent application was made public before Jazz’s patent application was made, so the timing of the processes favors Avadel in the court case.
The likely FDA approval, the high profit potential of FT218, as well as Avadel’s likely success in court are the key factors behind Needham analyst Ami Fadia’s bullish stance on AVDL. “Our investment thesis is three-fold,” she writes. “We believe that: (1) FT218 will be approved on PDUFA date October 15th, 2021 for the treatment of excessive daytime sleepiness (EDS) and cataplexy in narcolepsy, and will not be subject to a 30-month stay; (2) IP litigation initiated by JAZZ against Avadel shouldn’t delay FT218’s launch; (3) FT218 sales can grow to > $300M in 2023, and generate sales in the $400M+ range in the outer years on a non-risk-adjusted basis given its more convenient once-nightly dosing vs other sodium oxybates.” Fadia believes that the company will be able to initiate its commercial launch on schedule in early 2022.
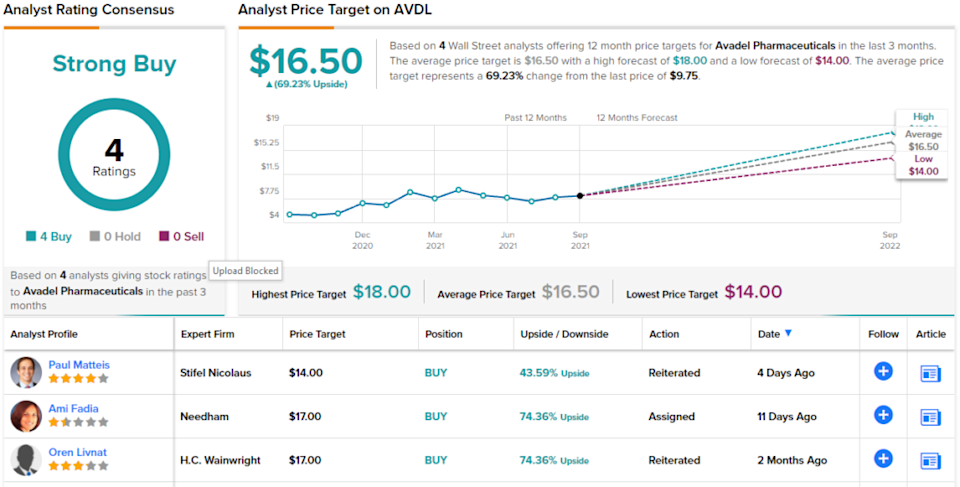
In line with this outlook, the analyst sets a Buy rating on AVDL stock, and her $17 price target suggests room for 73% share appreciation in the coming year. (To watch Fadia’s track record, click here.)
The unanimous Strong Buy consensus rating, based on 4 recent reviews, shows that Wall Street generally is wide awake to the potential here. The average price target of $16.50 implies a one-year upside of 69% from the $9.75 trading price. (See Avadel’s stock analysis at TipRanks.)
Eyenovia, Inc. (EYEN)
The second stock we’re looking at, Eyenovia, is a late-clinical stage ophthalmic research company; that is, Eyenovia is developing new medications for use in or on the eyes. The company is working to improve microdosing formulations of existing ophthalmic drugs through a highly precise, targeted delivery system.
The company has two drug candidates in advanced development. MicroLine, under investigation as a treatment for presbyopia – a gradual loss of the eye’s ability to focus on nearby objects – has successfully completed the Phase 3 VISION-1 study, and a second Phase 3 trial, called VISION-2, is scheduled for initiation by year’s end. Another drug candidate, MicroPine, is a treatment for nearsightedness in children. This drug has completed enrollment for the Phase 3 CHAPERONE study.
But the drug of interest here is MydCombi, a proprietary, first-in-class combination of pupil dilation drugs. MydCombi is intended as an anesthetic-free, microdosing agent for use in ophthalmic eye exams. This is a high-potential niche, as there are tens of millions of office-based eye exams performed annually in the US alone. In addition, an estimated 4 million cataract surgeries annually are likely to use the drug candidate as well.
The FDA is expected to make its decision on Eyenovia’s NDA for MydCombi shortly, with a PDUFA date set for October 28. A positive decision will clear the path for commercialization, targeting the extensive potential patient base.
Ahead of the big day, Ladenburg Thalmann’s Matthew Kaplan backs the drug’s chances and anticipates a successful market entry.
“We expect MicroStat (Mydcombi) NDA approval and market launch in the U.S. in 4Q21,” the analyst wrote. “The commercial strategy differs from traditional launches as Eyenovia will not hire a sales force; instead, they will maintain ~10 key account managers to initially focus on the largest practices…. With Eyenovia’s plan to price MicroStat on parity to the current eye drops based on their market research, and with an estimated 80 million dilated eye exams and ~4 million surgeries requiring dilation performed each year, we believe it could represent a significant market opportunity for MicroStat.”
Kaplan gives EYEN shares a Buy rating, along with a $19 price target that implies an impressive upside of 288% in the 12 months ahead. (To watch Kaplan’s track record, click here.)
This stock has slipped under the radar to a degree, and has only 2 recent reviews. But both are positive, making for a Moderate Buy consensus view. Eyenovia shares are priced at $4.90 and have an average target of $15.50, giving the stock a 216% upside potential. (See Eyenovia’s stock analysis at TipRanks.)

To find good ideas for healthcare stocks trading at attractive valuations, visit TipRanks’ Best Stocks to Buy, a newly launched tool that unites all of TipRanks’ equity insights.
Disclaimer: The opinions expressed in this article are solely those of the featured analysts. The content is intended to be used for informational purposes only. It is very important to do your own analysis before making any investment.
